A review of Veterinary Research in Epigenetics
 Saturday, August 23, 2014 at 12:00PM
Saturday, August 23, 2014 at 12:00PM Rebecca Zaremba, Ross University
Cases/Abstracts, Honorable Mention
For many years, millions of healthy women and their families have suffered from miscarriage, which is openly defined as the loss of a fetus under 20 weeks of age (The March of Dimes). The trauma of miscarriage often impacts entire families, from expectant mothers and fathers to siblings, grandparents, aunts and uncles. Many factors can cause miscarriage, and most of these are poorly understood. It is important to determine etiologies of miscarriage and it is also equally important to be able to understand that these tragedies do not disappear after the loss of the baby. Fortunately, the veterinary field has helped immensely in determining specific point mutations which are thought to be responsible for such tragedies in humans.
One of the long-term goals of the Lossie lab is to understand the genetic and epigenetic causes of miscarriage. In an effort to understand these mechanisms, we have characterized two lethal mutations in mice known as l11Jus1 (L1) and l11Jus4 (L4). L1 and L4 are two separate mutations in a gene called Notchless (Nle1), which is a component found downstream to the Notch pathway (Baumgarner et al. 2007). These two mutant lines survive through the blastocyst stage (Figure 1) and are able to successfully implant into the uterus. However, neither L1 nor L4 survive past implantation; they arrest prior to gastrulation, which eventually leads to an immature body.
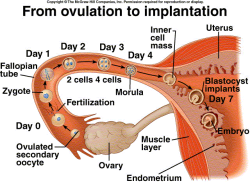
|
Figure 1. Implantation |
plan (Stern, 2004) and eventually results in miscarriage. The L1 and L4 alleles of Nle1 display gastrulation defects and apoptosis of the inner cell mass, which are phenotypes similar to those reported in a targeted disruption of the Nle1 gene (Cormier et al. 2006), strongly suggest that L1 and L4 are excellent systems to study the role of the Notch pathway in miscarriage (Baumgarner et al., 2008).
The Notch pathway is a major signal transduction pathway that is vital for cellular differentiation, development and cancer (Harper et al. 2003; Chiba 2006). There are 4 Notch receptors (Notch1-4) and 5 ligands (Delta-like1,3 & 4 and Jagged1 & 2) in mammals (Harper et al. 2003). Downstream targets include Hes1, Hes5 and Hes7, which are responsible for somitogenesis and the development of the nervous and immune system (Chiba 2006). Another set of target genes within the Notch Pathway (Hey X, Hey Y and Hey Z) aid in the development of the cardiovascular system (Chiba 2006). The Notch pathway is critical for embryonic development, as gene knockouts for Notch1, Notch2, Jagged1, Jagged2 and Delta-like 1, result in embryonic lethality at a time that corresponds to miscarriage in humans (Royet, 1998).
The objectives of the project were to better understand the role that Nle1 plays in miscarriage. Nle1 lies downstream of the Notch receptors and ligands, but upstream of the Hes and Hey genes, indicating that it potentially plays an important role in the amplification of Notch signaling between ligand/receptor binding and downstream target gene activation. We, therefore, hypothesized that mutations in Nle1 (i.e. L1 and L4) would impact gene expression levels of downstream target genes (Figure 2).
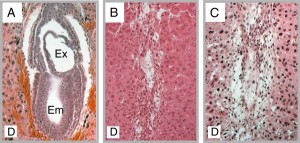
|
Figure 2. Mutant Phenotypes. H&E stained sections at E7.5 A. Wild type implantation site B. l11Jus1 implantation site. C. l11Jus4 implantation site. embryo (Em), extra-embryonic region (Ex), and maternal decidua (D) |
In order to address this hypothesis, we designed a set of experiments that compare global expression profiles in mutant embryos vs wild-type embryos. We will accomplish this by pooling mutant and wild-type blastocysts and performing microarray studies on RNA derived from the two sets of pooled embryos.
We mated our L1 heterozygotes to C57Bl/6J mice to generate L1/C57Bl/6J heterozygote animals. At this stage, half of the progeny should carry the L1 mutation. My role in the research was to identify which of the progeny carried the L1 mutation. In order to accomplish this goal, I collected tails from all of the progeny with which I isolated genomic DNA from, and diluted the DNA to an appropriate concentration for PCR. Following PCR, I purified the PCR products and sequenced each sample in order to identify which contained the L1 mutation. Genotyping the mice that would be used in the microarray experiment allowed the lab to get one step closer to finding the possible pathways that are affected by the mutation. Furthermore, these findings could then lead to the determination of the differences between L1 mutants and wild type embryos. During my time in the lab I was able to isolate genomic DNA from a number of progeny and identified many of the heterozygous individuals. The significance of determining the location of these mutations, as well as the identification of which other genes are mis-expressed in L1 mutants will help determine if women, who are prone to miscarriage carry mutations in the Nle1 gene. If they do, future studies can be designed to screen embryos for mutations in Nle1, and increase the likelihood of successful pregnancy. Although the success of these experiments can ultimately improve quality of reproduction, such accomplishments can also void not only the physical pain, but emotional pain as well.
Along with the attainment of improved human life, these successes can be applied to commercial and production animal businesses as well. This is evident because consistent studies could help in decreasing the amount of animals with Intra-Uterine Growth Retardation (IUGR), a leading cause of low birth weight and neonatal mortality in livestock (Wu, 2006). In return, animals would be able to reproduce healthier progeny and not be physically affected with the similar symptoms that are incurred by human women. This evidence can also help relieve the reproducing animal of any physical pain and can lead to a more economically stable commercial and production animal businesses.
Consequently, the continuation of research of genetic mutations will significantly decrease early mortality resulting from miscarriages and stillbirths. The achievement of these goals will also help alleviate any future emotional pain that could be incurred by a mother or family member and decrease monetary hardship faced by those involved in food animal businesses.
Bibliography
Baumgarner, Katherine (2008). Searching for an Early Embryonic Lethal Mutation.
pp. 8-9, 13, 15, 17
Bear, Christi (2002). Miscarriage. Focus on the Family. Retrieved July 9, 2008.
March of Dimes Foundation (2008). Quick Reference and Fact Sheets. March of Dimes Professionals and Researchers. Retrieved June 9, 2008. http://www.marchofdimes.com/
Miller, John L (2000). Mental Health Touches (everyone). Retrieved July 13, 2008.
http://www.athealth.com/
Royet, J., Bouwmeester, T., and M.Cohen, S. EMBO Journal. 1998;17: 7351-7360
Stern, Claudio D (2004). Gastrulation: From Cells to Embryo. Cold Spring Harbor, New York: Laboratory Press.
Wu, G., F. Bazer, J. Wallace, T. Spencer. 2006. Board-Invited Review: Intrauterine growth retardation: Implications for the Animal Sciences. Journal of Animal Sciences 84: 2316-2337.
Baumgarner, K. M., M. J. Cramer, et al. (2007). Searching for an Embryonic Lethal Mutation Associated with DNA Methylation. American Society of Human Genetics, San Diego, CA, American Journal of Human Genetics.
Chiba, S. (2006). "Notch signaling in stem cell systems." Stem Cells 24(11): 2437-47.
Cormier, S., S. Le Bras, et al. (2006). "The murine ortholog of notchless, a direct regulator of the notch pathway in Drosophila melanogaster, is essential for survival of inner cell mass cells." Mol Cell Biol 26(9): 3541-9.
Harper, J. A., J. S. Yuan, et al. (2003). "Notch signaling in development and disease." Clin Genet 64(6): 461-72.
 ross university in
ross university in  Cases/Abstracts
Cases/Abstracts 